To establish successful infections in a host, pathogens need to break through or evade the host’s immune system. Human immunodeficiency virus-1 (HIV-1), the causative agent of AIDS, is a master of this practice. The ability of HIV-1 to evade host immunity also contributes to viral persistence: it prevents host’s immune cells from acting competently on finding and killing HIV-1-infected cells. Therapeutic disruption of such viral manipulation is an attractive direction of antiretroviral drug discovery—it should revitalize the immune mechanisms against HIV-1 infections, which may possibly lead to a cure.
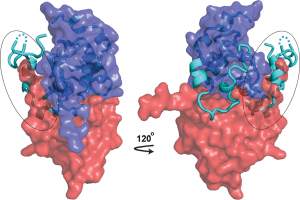
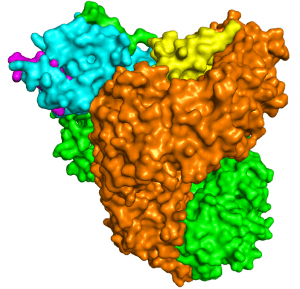
As structural biologists, we strive to contribute to this campaign by uncovering the mechanistic details—at atomic resolution—of immune evasion by HIV-1. A molecule of particular interest here is the HIV-1 accessory protein Nef, which disables multiple host immune mechanisms and thus plays a key role in viral pathogenesis (You can read a little story about Nef in this blog). By elucidating the structural bases of Nef’s cellular functions, we provide the biological insights, and inspirations, for Nef inhibition. Indeed, a second component of our research is to capitalize on our structural findings to develop such compounds; we do so through both high-throughput screening and structure-based rational design.
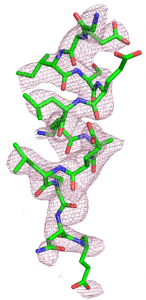
Our main research tools are macromolecular X-ray crystallography and cryo-electron microscopy (cryo-EM). In addition, people in the lab are also trained rigorously in molecular biology, protein expression and purification, protein biochemistry, development of in vitro assays, high-throughput screening for drug discovery, etc.